Around 5000 flights have been canceled in the US since January 2, 2022 due to the Omicron outbreak.
With more than 215% surge in 15 days, Omicron is de-normalizing the ‘new normal’ again!
Not only in the US, but the entire world is coping with the new variant.
Such an outbreak is quite challenging for the clinical trial industry. Even in normal times, this industry faces a lot of challenges.
For example, predicting the cost of the human trial phase.
Clinical trials signify one of the most essential mechanisms to convert scientific discoveries into clinical knowledge, assess new diagnostic or therapeutic regimens, and advance practice beyond the standard of care.
Before seeking FDA approval, the clinical trial companies need to have a clear picture of the future cost for specific clinical trials.
Cost is a serious issue in business. And pharmaceutical and biotechnology companies need to know the cost of clinical trials to plan their funding for the drug development programs.
The clinical trial phases are extremely complex and lengthy, especially during the human trial phase. Hence, companies need the right expenditure planning.
However, most companies struggle to understand the costs in the early stages of the clinical trial.
This article aims to help the CEOs, VPs, and chief executives of operations and sales to better understand the key ways to increase efficiencies and reduce the costs in clinical trials.
How to increase efficiency in clinical trials?
Before jumping into the solutions, let’s pinpoint the efficiency issues you face in clinical trials:
Slow and inefficient enrollment
There are multiple factors that can cause a slow enrollment of research subjects in any clinical trial process. These factors create operational inefficiencies in the clinical trial industry:
- Unnecessarily strict eligibility criteria
- Difficult trial requirement for visits and investigations
- Inflexibility of trial stipulations
- Safety monitoring and endpoint evaluation
Traditional Ink signatures
Especially during this pandemic, some trial processes get slow due to manual and traditional ink signatures that are required to approve each of the stages.
Operational delays
The clinical trial companies receive thousands of applications in each human trial phase. With conventional CPQ and CLM processes, your sales team finds it difficult to process all these applications.
Our Solutions
We study your existing processes and plan key transformation with fewer disruptions.
With our Salesforce and Conga partnership, we intervene your existing QTC, CLM, and CPQ processes with cloud transformation and migration.
We empower the clinical trial companies to gather and analyze all data in a centralized cloud location with authorized-based access.
We significantly reduce your quote and contract creation time.
For more information, schedule a free consultation call with us.
Costs in clinical trials
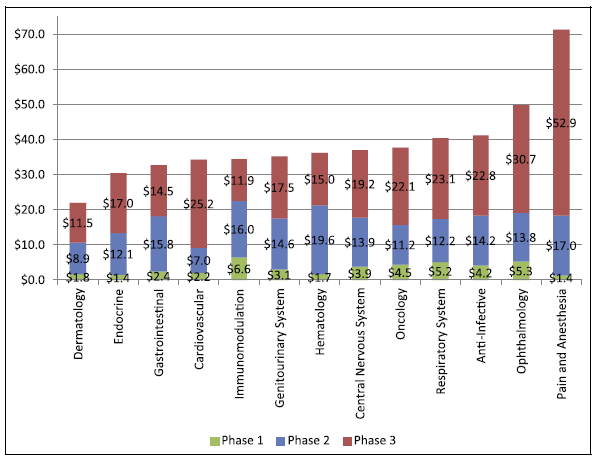
The costs of the total 3 clinical trials in the USA are within the range of US $1.4-6.6, 7.0-19.6, and 11.5-52.9 million respectively..
The two key factors involved in these costs are:
- Participant’s number
- Criticality of the clinical trial protocol.
The cost of phase 3 clinical trials in the US is estimated from USD 11.5 M up to USD 52.9 M, as shown here:
Source
Additionally:
- The average cost of manufacturing a drug for critical illness is around US $40,000 to 100,000.
- Manufacturing cost of a drug product batch falls in the average range of US $300,000 to 1 million.
How to reduce these exponentially huge costs?
We at CommerceCX re-engineer your existing processes with cloud-based QTC, CLM, and CPQ solutions. Our certified Salesforce team helps you with:
- Empower your sales team by suggesting the right combination of products as per your customer’s requirements.
- Automated applying bulk discounts to offer the right price along with the right product combination.
- Streamlines quote creation process with no error in quote and faster quoting system.
- Reduces back and forth in the contracts with a centralized process.
- Authorized teams can see the change requirements and do the changes on a single document.
- e-Signature secures your information and saves your time.
- Pre-defined approval rules accelerate your typical approval processes.
Now, you must wonder how these can help in reducing the cost? Let’s keep it simple.
- When we optimize your QTC process, the sales per customer get boosted from 10 to 15% with incremental revenue.
- Your sales cycle time reduces from 30 to 60%.
- Your Quote-to-Order cycle time reduces from 25 to 50%.
- Your order processing cycle time reduces from 20 to 40%.
- Your invoice generation time reduces by 10%.
- You get 20% improvement in creating error-free quotes.
- You get a 20-30% increase in cross-sell and upsell revenues.
Overall your sales team gets more time to close clinical trial deals by saving so much time. And when you save such an enormous amount of time, you increase your operational efficiency. Such revenue boosting also helps you reduce cost.
This is a predictive way of reducing cost in the clinical trial sector, as you don’t have many options left to decrease costs in all phases of trials.
Talk to us to know more about contract renewal.











