If you’ve ever even glanced at a request-for-proposal or a clinical trials contract, you might already understand why contract lifecycle management systems struggle to handle them. Contract research organizations are subject to intense regulation, scrutiny, and oversight and this level of complexity is hard for a base CLM to account for.
Just because it’s hard doesn’t mean it’s not worth it, though. CLM systems can automate simple processes and help streamline the contract lifecycle process, making everyone’s lives easier—but only if a system is implemented correctly. To do that, it’s important to consider what base CLM systems struggle with—and what to watch out for.
Difficult to track client types and locations
In basic contracting, a legal entity is anyone who can legally enter into a binding contract. For individuals or even small businesses, the status of a legal entity is straightforward. Basic contract lifecycle management systems like Conga are fully capable of dealing with these simple legal entities.
The problem is, when has anything in clinical trial management been simple?
Healthcare is a global industry, and hundreds of countries are invested in the outcomes of clinical research, whether it be homegrown or conducted by a contract research organization. Because of this, many clinical trial companies have developed global presences out of necessity. IQVIA has locations everywhere from Argentina to Estonia to Vietnam; LabCorp labs can be found in Auckland and Osaka and Zurich; the lists go on and on.
With so many different countries come so many different regulations and expectations, and client types. Clinical research organizations can contract with private companies, public universities, and even governments—and each type of client has its own regulatory and ethical bodies to keep track of and report to.
An out-of-the-box solution simply can’t account for this level of complexity, and without experienced aid, many CROs struggle to even visualize how their CLM systems might look.
Complicated and changing contracts make it difficult to keep up
We’ve just discussed how complicated legal entities can be to keep track of—but the complications of their rules and regulations grow exponentially with every additional country a CRO does business in. This means that contract terms can change frequently during drafting and even sometimes after the contract has already been signed.
Contracts for clinical trials are complicated beasts. First, you have to come up with the overarching contract, which encapsulates the goals of the research, the regulations to be followed, the legal entities participating, etc. Even if you create standard templates, clinical research organizations—especially smaller ones—must often adhere to contracts on customer paper, which can require major changes per contract that a template can’t accommodate.
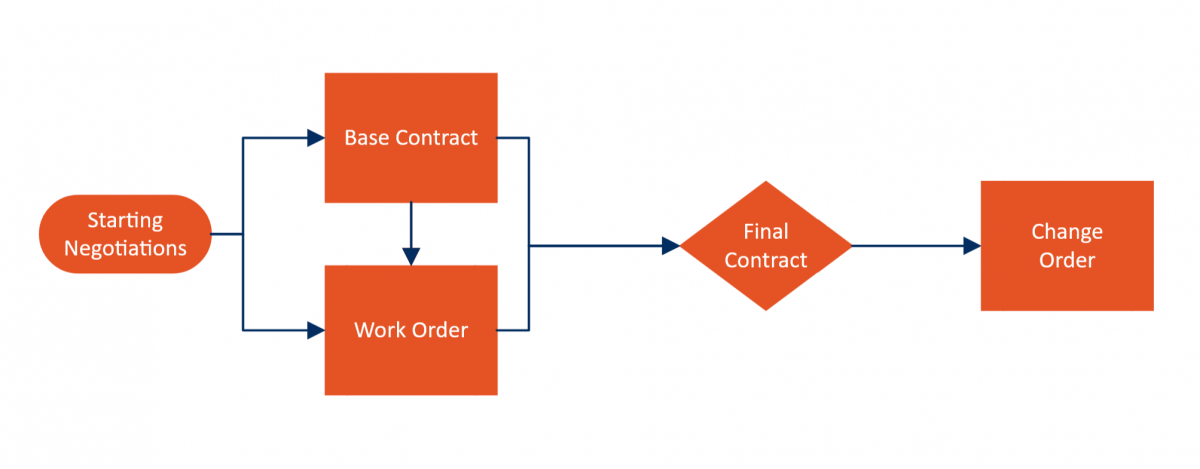
And that’s just the main contract. The actual work often gets broken down into work orders, which follow the rules laid out by request-for-proposal but also delineate the specific work the CRO will be performing and how that work will be reported to the client.
If that weren’t enough, clinical trials also frequently make discoveries in data that result in avenues of research that hadn’t even been considered when the contract was written. CROs then have to write change orders to modify the existing contract to allow them to conduct broader research—like when many infectious disease research projects diverted funds from their primary research to COVID-19 in 2020.
CLM systems are built to cater to basic contracts and struggle to account for the constantly changing terms that permeate a request-for-proposal throughout its lifecycle. And when you need to make a quick change, sometimes it feels easier to stay manual than to try and digitize.
Parallel quoting and contracting complicate process
The cycle from a request-for-proposal to a trial start date is a long one. Both salespeople and clients want to make sure that process goes as quickly as it can so that researchers can get down to business and start gathering meaningful data. Because clinical trials must incorporate payment and budget into their contracts, quoting and contracting often happen in parallel—a legal team works with a sales team and the client to draw up a contract, while the salesperson works to put together a quote.
This means salespeople are quoting while contract teams are still trying to manage contract parameters like payment terms and risk. If all goes according to plan and everyone coordinates effectively, the CRO can provide a quote to the client-agency as soon as the contract is signed.
This synergy is hard to accomplish in a digital CLM system. Good use of automation can accelerate certain processes on both sides: a good pricing engine can track a listed contract price and apply it whenever it’s updated, a library of legal clauses can help craft a contract without correspondence with legal, etc. But these automated add-ons create more of a coordinated system than a parallel system. They still require a great deal of manual communication and labor to achieve any kind of parallel output, plus the user must incorporate learning the new system. With more training for the same reward, it can seem easier to do it the old-fashioned way.
CLM can do a lot to improve clinical trials companies’ day-to-day, but not knowing what obstacles to consider can do more harm than good. To find out how CommerceCX has helped clinical trials companies overcome these obstacles, learn how we helped IQVIA resolve some of their biggest contract woes.











